
The difference is a measure of the nuclear binding energy which holds the nucleus together. Mass was no longer considered unchangeable in the closed system. Note that, it was found the rest mass of an atomic nucleus is measurably smaller than the sum of the rest masses of its constituent protons, neutrons and electrons. For 63Cu the atomic mass is less than 63 so this must be the dominant factor. A nucleus with greater binding energy has a lower total energy, and therefore a lower mass according to Einstein’s mass-energy equivalence relation E = mc 2.

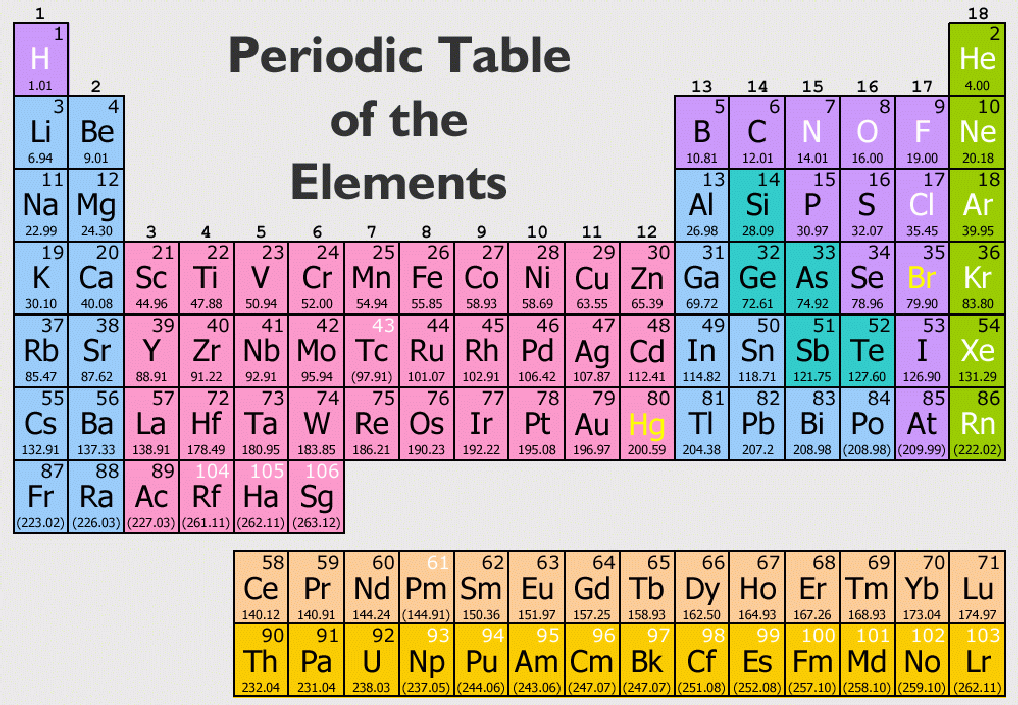
There are two reasons for the difference between mass number and isotopic mass, known as the mass defect: For example, 63Cu (29 protons and 34 neutrons) has a mass number of 63 and an isotopic mass in its nuclear ground state is 62.91367 u. For other isotopes, the isotopic mass usually differs and is usually within 0.1 u of the mass number. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.įor 12C the atomic mass is exactly 12u, since the atomic mass unit is defined from it. One atomic mass unit is equal to 1.66 x 10 -24 grams. The unit of measure for mass is the atomic mass unit (amu). Units of measure have been defined for mass and energy on the atomic scale to make measurements more convenient to express. The size and mass of atoms are so small that the use of normal measuring units, while possible, is often inconvenient. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance. The atomic mass is carried by the atomic nucleus, which occupies only about 10 -12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.
Information for the timeline was compiled from () data, in particular Wikipedia's chemical element discoveries ().The atomic mass is the mass of an atom.
#Chemiccal element 39 free
This timeline was made using (/), a free online service for producing interactive timelines that can be shared on the web. We hope this multimedia timeline will be a valuable resource for educators who want to teach their students about the history of chemical elements, and also for anyone who is interested in the history of scientific discovery. The timeline offers an alternate visual perspective on the chemical elements, in contrast to the famous () devised by Russian chemist (). Here, you can find out when all of the 118 known chemical elements were discovered and read about the pioneering men and women who identified them - people like () and () Curie, who won Nobel prizes for their discoveries of Radium and Polonium.

Welcome to ChronoFlo's interactive timeline of chemical element discoveries.


 0 kommentar(er)
0 kommentar(er)
